Полиизоцианат МДИ
Узнайте цену, наличие и характеристики
Полиизоцианат (Метилендифенилдиизоцианат (МДИ), дифенилметандиизоцианат) — ароматический диизоцианат. Общеизвестен в виде трех изомеров: 4,4-МДИ — наиболее широко используемый изомер, также известен как чистый МДИ и 2,2-МДИ, 2,4-МДИ.
Полиизоционат это темно-коричневая жидкая смесь 4, 4 — дифенилметандиизоцианата (MDI) с изомерами и гомологами.
В производстве пенополиуретана (ППУ) МДИ чаще обозначается как «компонент Б» системы, образующей полиуретан реагируя с «компонентом А».
Хим.формула C15H10N2O2
Синонимы и международное название:
полиметан полифенил изоцианат, полиметилен полифенил изоцианат, дифенилметандиизоцианат, полимерный дифенилметан диизоцианат, дифенилметан-4.4′-диизоцианат, 4,4′-диизоцианатодифенилметан, 1-изоциано-4-[(4-изоцианофенил)метил] бензол, компонент В.
WANNATE PM-200 — Жидкость коричневого цвета.
Применяется в качестве компонента B при производстве пенополиуретана. Применяется в производстве жестких пенополиуретановых теплоизоляционных материалов; также используется в изоциануратной пене, красках, клеях, конструкционной пене и тд.
Синонимы и международное название: Полиолы, Силиконовые стабилизаторы, Катализаторы, Трихлорпропилфосфат. 6. Полиметилен полифенилен изоцианат 8. Аналогами являются: DESMODUR 44V20L, Lupranate M20S.
Полиизоцианат МДИ
CAS № 9016-87-9
Полиизоцианат МДИ
Cosmonate M200
Полиизоцианат МДИ
WANNATE PM-200
| NCO, % | 30.00-32.00 |
| Внешний вид продукта | Коричневая жидкость |
| Вязкость (25°C), Па*с | 150-250 |
| Кислотность, % | 0.1 max |
| Молекулярная масса, г/моль | 350-400 |
| Точка замерзания, °C | — 20max |
| Точка кипения, °C | 200-208 |
| Точка самовоспламенения, °C | 600 min |
| Удельная масса (25°C) | 1.230-1.250 |
| Внешний вид | Коричневая жидкость |
| Вязкость | 150-250 (25℃)/(мПа·с) |
| Содержание NCO | 30,5-32,0 % |
| Плотность | 1,220-1,250 (25℃) / (г/см3) |
| Содержание кислоты | ≤0,030 % |
| Гидролизуемый хлор | ≤0,20 % |
Полиизоцианат МДИ: применение
- Производство пенополиуретанов, пенолиуретановых пен, клеев, лаков и покрытий
- Производство покрытий CASE (Coating, Adhesives, Sealants, Elastomers) адгезивов, герметиков и эластомеров
- Производство полиуретановых полов
- Производство преполимеров, уплотнительных материалов
Полиизоцианат МДИ: упаковка
Металлические бочки по 250 кг.
Получите экспертную консультацию
по продукту и условиям поставки
Полиизоцианат МДИ: ваш менеджер по продажам
Юлия Маслова
100% гарантия качества
Бесплатные образцы
В наличии на складе и под заказ
Консультация эксперта
Доставка по РФ и СНГ
Рассчитайте стоимость онлайн
Выберите
марку →
Полиизоцианат МДИ
Cosmonate M200
Полиизоцианат МДИ
WANNATE PM-200
C этим товаром также покупают:
Сообщение отправлено!
Спасибо за Ваш интерес к нашей продукции. Ваш запрос будет рассмотрен нашими
менеджерами в самое ближайшее время.
Что-то пошло не так 
Спасибо за Ваш интерес к нашей продукции. К сожалению по техническим причинам
Ваша заявка не отправлена. Попробуйте чуть позже, либо свяжитесь с нами по контактным телефонам!
- Выдавший орган
- Роспотребнадзор (Федеральная Служба)
- Типографский номер бланка
- 12102
- Продукция
- Полиметиленполифениленизоцианат (синонимы: Эфир изоциановой кислоты с полиметиленполифениленом, полимер дифенилметандиизоцианата; торговые названия: Полиметиленполифенилполиизоцианат, входит в состав продукта Корундинат ПМ. CAS 9016-87-9)
- Свидетельство выдано
- на основании заключения об опасных свойствах вещества № 07/23 от 21.03.05 г.; Информационной карты потенциально опасного химического и биологического вещества Российского регистра потенциально опасных химических и биологических веществ МЗ РФ серия ВТ № 002702 от 10.03.2005 г. Действительно до 10.03.2008 г. Для регистрации с постоянным сроком действия необходимо представить следующую информацию: гигиенические нормативы в воздухе рабочей зоны; воде водных объектов хозяйственно-питьевого и культурно-бытового водопользования; воде водных объектов, имеющих рыбохозяйственное значение; методы определения в воздухе рабочей зоны и воде
- Получатель
- ООО «Корунд», 606000, Нижегородская обл., г. Дзержинск, ул. 1 Мая, 1 (Российская Федерация)
- Область применения
- для использования в химической, текстильной, кожевенной промышленности; в строительстве
- Фирма-изготовитель
- продукция изготовлена ООО «Корунд», 606000, Нижегородская обл., г. Дзержинск, ул. 1 Мая, 1 (Российская Федерация)
- Нормативная документация
- ГОСТ 12.1.007-76 «ССБТ. Вредные вещества. Классификация и общие требования безопасности» ГН 2.1.6.1339-03 «ОБУВ загрязняющих веществ в атмосферном воздухе населенных мест» ГН 2.2.5.1313-03 «Предельно допустимые концентрации (ПДК) вредных веществ в воздухе рабочей зоны»
- Гигиеническая характеристика
-
Малоопасное вещество при внутрижелудочном и чрезвычайно опасное при ингаляционном поступлении в организм 4 и 1 класс опасности соответственно. Обладает слабой способностью к кумуляции. Раздражает кожу и слизистые оболочки глаза. Обладает сенсибилизирующим, эмбриотоксическим и тератогенным действием. ОБУВатм.в. 0,02 мг/куб.м Вещество не нормировано в воздухе рабочей зоны и воде водоемов. Осуществлять контроль в воздухе рабочей зоны по незаполимеризованному мономеру 1,1′-метиленбис(4-изоцианатбензолу) ПДКраб.з. 0,5 мг/куб.м, пары+аэрозоль, 2 класс опасности, А (вещества, способные вызвать аллергические заболевания в производственных условиях), + (требуется специальная защита кожи и глаз).
Документ получен с сервера поиска по Реестрам Роспотребнадзора и санитарно-эпидемиологической службы России
Wood: Adhesives
A.H. Conner, in Encyclopedia of Materials: Science and Technology, 2001
2.3 Isocyanate Resin Adhesives
Isocyanates are important industrial chemicals used in injection molding and for the production of polyurethane foams. All isocyanates of industrial importance contain two or more isocyanate groups (


Figure 5. Synthesis of polymeric diphenylmethane diisocyanate (PMDI).
These covalent bonds, to the extent that they form, act to anchor the polyurea to the wood and help bridge the gap between pieces of wood. As an adhesive, PMDI has several reported advantages:
- (i)
-
Because water is needed to form the polyurea that acts as the polymeric adhesive material, PMDI can be used with wood that has a higher moisture content than that usually employed with other adhesives. This eliminates part of the energy needed to dry the wood prior to bonding. It also means that the wooden materials are bonded closer to the moisture conditions at which they will be used. This eliminates a large portion of the stress that develops in the bonded material as it regains moisture after bonding, thereby imparting greater dimensional stability.
- (ii)
-
Because of the greater reactivity, PMDI can be cured at a lower temperature. This eliminates a portion of the energy required to form the bonded assembly.
- (iii)
-
Less PMDI is needed on a weight basis to form a bonded material with acceptable properties.
- (iv)
-
There are no formaldehyde emissions associated with the adhesive.
There are also some disadvantages associated with the use of PMDI as an adhesive:
- (i)
-
Isocyanate adhesives cost more than PF and UF resin adhesives.
- (ii)
-
PMDI is an excellent adhesive for metals as well as wood. Thus, MDI-bonded boards (e.g., oriented standard board) tend to adhere to the press platens. This problem has been overcome by the use of release agents. A second approach has been the layering of materials during panel formation, with those layers that come into contact with the platens being bonded with a PF adhesive. Thus, the exterior of the panels is bonded with PF, but the interior of the panel is bonded with MDI.
- (iii)
-
Isocyanates, like other adhesive resins, are toxic chemicals. MDI is a respiratory irritant that produces asthma-like symptoms. Individuals can also become sensitized to isocyanates. Although MDI has a high vapor pressure, precautions should be taken to eliminate the inhalation of aerosols or dust particles coated with MDI.
Surprisingly, MDI can also be used in admixture with water. One method for doing this is to emulsify the MDI in water, giving an adhesive resin known as emulsified MDI (EMDI). It is thought that a thin film of polyurea is formed around each MDI droplet. Water does slowly permeate the polyurea barrier, so EMDI has a limited shelf life. A second method is to chemically block the isocyanate groups, such that the reactive isocyanate groups are reformed on heating. The advantage of using MDI in water is that it gives a resin system with a viscosity essentially that of water. Thus, the resin can be applied to wood chips with conventional equipment.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B0080431526017344
Isocyanates
Robert Kapp, in Encyclopedia of Toxicology (Second Edition), 2005
MDI – 4,4′-Methylenediphenyl Diisocyanate
- •
-
Chemical Abstracts Service Registry Number: CAS 101-68-8
- •
-
Synonyms: 4,4′-Diphenylmethane diisocyanate; Methylene bis(4-phenyl isocyanate); Methylene di-p-phenylene ester of isocyanic acid
- •
-
Chemical Formula: C15H10N2O2
Uses
4,4′-Methylenediphenyl diisocyanate (MDI) is used to produce polyurethane foams.
Background Information
MDI is a light-yellow fused solid or it may occur in crystalline form.
Exposure Routes and Pathways
Inhalation and dermal exposure can occur during the manufacture and use of MDI. Workers and individuals in close proximity to the plant may inhale emissions from urethane foam production manufacturing facilities.
Acute and Short-Term Toxicity (or Exposure)
Acute inhalation exposure may result in sensitization and asthma in humans. Dermal contact with MDI resulted in dermatitis and eczema in plant workers. Animal studies revealed skin and eye irritation in rabbits, extreme toxicity by inhalation and moderate toxicity by oral ingestion in rodents.
Chronic Toxicity (or Exposure)
Chronic inhalation exposure to MDI is one of the leading causes of asthma in plant workers. In addition, chronic inhalation exposure can cause dyspnea, immune disorders as well as nasal and lung lesions. EPA has set the RfC for MDI at 0.0006 mg m−3 based upon irritation of nasal membranes in rodents. EPA has not established an RfD for MDI.
Reproductive Toxicity
No information is available in the reproductive or developmental effects of MDI in humans; however, some effects (decreased placental and fetal weights and increased skeletal variations) were noted in a rat study.
Carcinogenicity
No information is available on the carcinogenic effects of MDI in humans. Animals exposed to polymeric MDI were reported to increase the incidence of pulmonary adenomas. EPA has classified MDI as a group D (not classifiable as to human carcinogenicity).
Exposure Standards and Guidelines
The (US) OSHA PEL 8 h TWA is 0.02 ppm (0.2 mg m−3). The (US) NIOSH REL is 0.005 ppm (0.05 mg m−3) as a 10 h TWA, and the NIOSH IDLH value is 75 mg m−3.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B0123694000005378
Polymers for a Sustainable Environment and Green Energy
R. Höfer, in Polymer Science: A Comprehensive Reference, 2012
10.22.4 Rheology, Thickening, and Flow
The consistent control of flow properties is of the utmost importance in liquid polymer systems and throughout the manufacturing and application processes of all types of paints and lacquers. Additives that aid in controlling the rheology of a paint vehicle mostly work by structuring the liquid phase or on their own by forming three-dimensional networks and swelling or by interaction with dispersed phase particles via associative mechanisms and adsorption.
Rheology control by swelling of three-dimensional networks in organic and solvent-borne and solventless coatings, adhesives, and sealants50a is realized by the following:
‘Fumed silicas’ (also known as pyrogenic silica or fumed silicon dioxide) are produced by the vapor phase hydrolysis of silicon chlorides in a hydrogen-oxygen flame:
2H2+O2+SiCl4→SiO2+4HCl
The combustion process creates silicon dioxide molecules that condense to form particles. These primary particles (particle size 7–40 nm) collide, attach, and sinter together. The result of these processes is a three-dimensional secondary structure of branched chain aggregate with a length of approximately 0.2–0.4 μm. Once the aggregates cool below the fusion point of silica (1710 °C), further collisions result in the formation of a tertiary structure (agglomerates) by mechanical entanglement of the chains. The three-dimensional silica structures easily swell up with solvent and resin and contain the vehicle within their structure. The structures are broken with shear (which means that system viscosity falls over time) and reform again when shear is reduced or stopped, causing the viscosity to rise. Because the breakdown at a given shear rate continues with time, and with time, it rebuilds its structure, which is called thixotrope.50b
‘Treated silicas’, that is, hydrophobic silicas, are surface modified by grafting inert functional groups such as trimethoxyoctylsilane, hexa-methyldisilazane, alkyl-chlorosilanes, and oligomers or polymers such as PDMS to silanol groups on the particle surface of hydrophilic silicas.51
‘Organoclays’52 are made from naturally occurring smectite minerals with a high cationic-exchange capacity, more particularly from brentonite, montmorillonite, or hectorite. The cations adsorbed on the laminar plate surfaces can be readily substituted by organic cations, more particularly by quaternary ammonium compounds (QACs) such as dioctadecyldimethylammonium cations. Such hydrophobically modified clays are solvent swelling, which makes them suitable as rheology additives for solvent-borne coatings. Environmentally benign organoclays for applications such as viscosity promoters in flowable oil drilling emulsions alternatively use esterquats instead of QACs to achieve hydrophobic modification.53
‘Castor waxes’ are fully organic waxes such as hydrogenated (hardened) castor oil (HCO) or N,N′-ethylene bis(12-hydroxy stearamide) which both swell by end-to-end agglomeration of primary wax particles via hydrogen-bonding.
Rheology control by swelling of three-dimensional networks is also practiced in aqueous coating systems. However, the rheology control agent in waterborne paints is almost always a water-soluble natural or synthetic polymer. The swelling-type rheology control agents are modified natural hydrocolloids such as hydroxyethyl cellulose (HEC), hydroxyethyl or hydroxypropyl starch, carboxymethyl cellulose, and synthetic polymers, more particularly acrylic acid homo- or copolymers. Hydrocolloids like HEC, when dissolved in water, thicken the water phase depending on MW, concentration, and temperature through interaction with the aqueous phase (i.e., association of water molecules alongside the polymer chain and formation of hydrogen bonds) and by entanglement of the polymer chains (Figure 16).
Figure 16. Thickening mechanism of hydroxyethyl cellulose (HEC).
Acrylic homopolymers are manufactured by solution polymerization in water. Copolymers with ethyl acrylate (EA), butyl acrylate, and/or higher alkyl (meth)acrylates like stearyl methacrylate, for example, are made by emulsion polymerization or by miniemulsion copolymerization.54 Such (meth)acrylic polymers (called ASE, i.e., alkali-soluble emulsions or alkali-swellable emulsions) are substantially insoluble in the acidic state. They only become soluble in the neutral state and at alkaline pH.55 When the medium is neutralized with a base, an ionic repulsion mechanism is induced between the various carboxylate groups on the polymeric chain (Figure 17). The emulsion clarifies and each emulsion particle swells greatly. The ionized groups polarize a large number of water molecules, leading to the increase in viscosity of the medium.56 The concentration of acid groups, the Mw, and the degree of cross-linking of the polymer are important in determining rheology and thickening efficiency of ASEs.
Figure 17. Thickening mechanism of alkali-swellable acrylic thickeners.
Hydrophobic modification of natural or synthetic hydrocolloids results in the formation of polymer surfactants. They are often referred to as hydrophobically modified water-soluble polymers (HM-P), associative polymers, or associative thickeners.57 The amount of hydrophobic side chains is quite small and can vary from 1 to 5 mol.%. The amphiphilic character of such polymer surfactants brings them to associate in aqueous solution and form micelles or adhere on interfaces with latex particles and pigments forming mixed micelles with latex-stabilizing emulsifiers and pigment dispersants. By this means, they effect rheology control in dispersed systems via ‘micelle bridges’ (Figure 18). Additionally, chain entanglement, associations between hydrophobic side groups, and associations of hydrophobic segments of the polymer backbone cause the increase of the viscosity to the aqueous solution.58
Figure 18. Associative thickening mechanism.
Associative thickening agents are grouped according to their chemistries:
‘Hydrophobically modified cellulosics’ cover hydrophobically modified hydroxyethyl cellulose (HMHEC) (Figure 19) and hydrophobically modified ethylhydroxyethyl cellulose (HMEHEC). HEC is manufactured by alkalization of cellulose and subsequent reaction with ethylene oxide. For the synthesis of EHEC the alkalized cellulose is modified by a reaction with ethylene oxide and then with ethyl chloride. Hydrophobization occurs by reacting HEC or EHEC with a C10–C18 alkylchloride or α-epoxide.59 The HMEHEC obtained in this way is an example of a comb-like HM-P. It has hydrophobic groups grafted along the water-soluble EHEC backbone. Only a small amount of hydrophobic groups are required to make the celluloseether associative. In a similar way, associative hydrophobically modified non-ionic polygalactomannan ethers, such as hydrophobically modified hydroxypropyl guar can be made (Chapter 10.08).
Figure 19. Hydrophobically modified hydroxylethyl cellulose (HMHEC).
‘Hydrophobically modified alkali-swellable emulsions’ (HASE) are dispersions of water-insoluble acrylic polymers in water. HASE thickeners contain a balance of carboxylic and acrylate ester groups so that the polymer is stable as a latex at acid pH, but dissolves and expands at pH > 6. They are typically prepared by emulsion polymerization as terpolymers of EA, methacrylic acid (MAA), and an associative macromonomer containing a long-chain hydrophobic group such as nonylphenol (EO)x(methacrylate) or fatty alcohol(EO)yacrylate (Figure 20).
Figure 20. Molecular structure of a hydrophobically modified alkali-swellable emulsion (HASE) polymer.
‘Hydrophobically modified ethoxylated urethanes’ (HEUR) are based on a nonionic water-soluble PUR. The MW normally is between 10 000 and 50 000. HEUR typically contain
- –
-
at least two hydrophobic terminal segments like lauryl, stearyl, or nonylphenyl;
- –
-
several hydrophilic segments such as polyethylene glycols; and
- –
-
urethane groups derived from diisocyanates such as 4,4′-diphenyl methane diisocyanate (MDI), hydrogenated MDI (H12MDI), xylylene diisocyanate (XDI), isophoron diisocyanate (IPDI), m-tetramethylxylene diisocyanate (TMXDI), tolylene diisocyanate (TDI), or dimer fatty acid diisocyanate (DDI).
HEUR have originally been applied as thickening agents in pigment pastes for textile printing.60 A typical example is a TDI-polyethylene glycol prepolymer capped with dodecyl isocyanate:
R-N(H)-C(O)-[Ex-DI]-E-OC(O)N(H)-R
where R is the hydrophobic end-group (dodecyl), E is CH2-CH2-O-, and DI is diisocyante.61 Besides such diblock HEUR thickeners, branched star-like HEUR bearing three or more hydrophobes per molecule and comb-type polymers with random hydrophobe placement have been claimed. The latter HEUR can be realized by the free-radical copolymerization of isocyanatoethyl methacrylate with non-isocyanate-containing monomers.62
‘Hydrophobically modified polyethers’ (HMPE) are, indeed, a category of HM-P that includes HEUR as a special case. HMPE comprise
- •
-
linear hydrophobically end-capped diblock polyethers with m-dichloromethyl benzene as difunctional nucleus (Figure 21),
Figure 21. Diblock hydrophobically modified polyether (HMPE).
- •
-
modified polyethers with three hydrophobic end-groups (Figure 22) manufactured, for example, in a three-step reaction of nonyl-phenol polyglycolether with epichlorohydrine, ring opening of the oxirane group by another nonylphenol polyglycolether, and capping the sec. hydroxyl group by reaction with octyl chloride,
Figure 22. HMPE with three hydrophobic end-groups.
- •
-
star-shaped polymers having more than three hydrophobically end-capped polyether arms (Figure 23).63
Figure 23. Star-shaped HMPE.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780444533494002739
Metallic, Ceramic, and Polymeric Biomaterials
R.S. Ward, R.L. Jones, in Comprehensive Biomaterials II, 2017
1.26.4.2.1.1 Adipate esters
Adipates are saturated polyesters that exhibit strength and durability, excellent tear and abrasion resistance, resistance to solvents and resistance to oxidation and UV light degradation. Depending on the hard-segment content and other substitutions, hydrolytic stability can vary from good to marginal: microorganisms have been isolated that can use adipic acid-based PUs as their sole source of carbon and nitrogen.79
An Estane polyurethane, is a commercially available random copolymer consisting of poly(butyleneadipate) (PBA) and butane diol soft segments formed from adipic acid and MDI and BDO hard segments.80 It has been used in a number of biomedical applications and has been evaluated for orthoplastic applications.81 Polymers including PBA soft segments are clearly not suitable as biostable polyurethanes due to the hydrolytic instability of the soft segment. They may be candidates of use in bioresorbable polymers but appear to be less desirable than polylactones (see Section 1.26.4.2.1.2).
Recently, Pierce et al.82 designed a series of completely amorphous poly(ester urethane) based on adipic acid and β-hydromuconic acid that has a wide range of mechanical properties. In kinetic analyses these materials were found to have extremely fast linear degradation profiles with little or no cytotoxic response.
For improving surface properties, Ming and Ping83 treated biomedical poly(ester)urethanes based on polyester adipate soft segments with low-powered gaseous CO2, O2, NH3, and SO2 plasmas, which resulted in the incorporation of oxygen-containing groups, nitrogen-containing groups and sulfur-containing groups. They found an increase in the polar character resulting from increased wettability for all the treated surfaces.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780081006917001798
Biomedical polymers
ROBERT G. HILL, in Biomaterials, Artificial Organs and Tissue Engineering, 2005
Polyurethanes
Polyurethanes are polymers that contain the urethane group.
A large number of urethane polymers exist with widely different physical and biological properties. The urethane grouping can be considered as resulting from the reaction of an isocyanate and an alcohol:
The polyurethane is then represented by:
There is a wide variety of polyurethanes due to the numerous possibilities for R and R′. R is typically an oligomeric (molar mass 200–500) hydroxylterminated polyether or polyester. Generally, synthesis is a two-stage process involving the preparation of a low molar mass pre-polymer followed by chain extension and/or cross-linking. Common pre-polymers are based on 2,4-toluene diisocyanate (TDI) or 4,4′-diphenylmethane diisocyanate (MDI). Polyether urethanes are usually based on polytetramethylene oxide (PTMO), polypropylene oxide (PPO) and polyethylene oxide (PEO). Polyester urethanes are typically based on polycaprolactones.
Chain extension may be performed by glycols or diamines. The nature of the chain extender is very important in that it determines chain flexibility and modulus. It is also possible to have urea groups –NH–CO–NH– and the urethane group –NH–CO–O–. Most polyurethanes for medical use are two-phase block copolymers (also termed segmented polyurethanes). The polyester or polyether glycol forms the soft segment and matrix phase.
In the early use of polyurethanes, acute reactions were observed on implantation. Most of the studies were done on commercial products rather than on well-characterised materials. The reactions observed are now believed to be due to in vivo ester hydrolysis of polyester urethanes. Problems have also occurred with rejection of breast implants coated with polyester urethanes due to degradation of the urethane. Segmented polyether urethanes are preferred because of their greater stability and lack of susceptibility to hydrolysis. Commercial examples include Biomer®, Pellethane® and Tecoflex®. Segmented polyether urethanes have good haemocompatibility and are one of the preferred polymer types for blood contacting devices.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9781855737372500109
Ring-Opening Polymerization and Special Polymerization Processes
D. Bogdal, in Polymer Science: A Comprehensive Reference, 2012
4.39.3.2.2 Polyurethanes
Owing to high versatility of the polyurethane raw materials, a variety of products with diverse structures and polymer matrix from flexible to rigid, especially cellular materials, are manufactured. In the middle of the 1980s, crosslinking of polyurethane resins was performed by means of pulsed microwave irradiation.103 The thermal behavior of an ethyl acetate solution of two prepolymers, triisocyanate (Desmodur L75) and polyester–polyalcohol (Desmophen 800), was investigated together with the formation of polyurethane coatings from the same mixture and film hardness as a function of different pulse regimes. The average power of microwave irradiation was 30 W and the pulse period varied from 2 to 200 ms so that the pulse time was varying from 50 μs to 30 ms.
The variations of the maximum temperature (Tmax) with peak power were reported where each curve was related to a pulse period. All curves were issued from the same point, corresponding to the reference continuous wave, that is, power 30 W with Tmax 70 °C. Starting from this point, Tmax always increases with pulse power (Figure 42). Thus, it was concluded that the energy transfer by pulse microwaves is more efficient than by continuous irradiation, and microwave-cured polyurethane films were very much harder than oven-cured materials.
Figure 42. Peak pulse power dependence of the maximum temperature Tmax (second parameter: pulse period).
Reproduced with permission from Jullien, H.; Valot, H. Polymer 1985, 26, 506.103
The crosslinking of polyurethane resin composed of diisocyanate derived from 4,4′-diisocyanate diphenylmethane and a low-viscosity polyethertriol was also investigated under microwave conditions.104 The reactions were carried out without a catalyst and led to final networks with mechanical properties least equivalent to those prepared under conventional conditions. For example, the average elasticity modulus determined from uniaxial compression with samples (25 mm of height and 12.5 mm of diameter) was equal to 3120 MPa for curing under microwave conditions (1 h at 20 W) and 2810 MPa for conventional curing in an oven (60 °C for 8 h).
The application of microwave irradiation is expected to help in the preparation of environmentally benign renewable components for polyurethane foams.105,106 The vegetable oil-based polyols are examples of such components that can be used to obtain rigid polyurethane foams that possess satisfied properties for use as thermal insulating materials.
For this purpose, for the preparation of rapeseed and linseed oil-based polyols a two-step process was applied.107 In the first step, the double bonds of the unsaturated triglycerides were transformed into oxirane rings with acetate peroxyacid to form epoxidized oil. In the second step, the ring opening reactions of the epoxy groups with monoethylene glycol (MEG) or DEG afforded the oil-based polyols. For rapeseed and linseed oils, it was reported that microwave irradiation can be applied for both steps of the process. In comparison to the processes under conventional conditions, reduction of the reaction time of epoxidation reaction (ca. 60%) and, then, the hydroxylation step (ca. 75%) was observed.
In turn, blowing and curing processes of rigid polyurethane foams under microwave irradiation were also investigated.108 The polyurethane foams were blown inside a microwave reactor with continuous microwave power regulation. The foams were prepared in one step, that is, isocyanate was added to polyol premix with additives, and the mixtures were stirred for 10 s. The foaming processes were carried out as a free rise in the open mold and the mixtures were expanded freely in the vertical direction on a square area of 20 cm × 20 cm in polypropylene molds at ambient temperature (ca. 20 °C). In the second case, the polypropylene molds were placed inside a microwave reactor. The results reflected the dependence of foam properties on such parameters of microwave irradiation as power and time, and the application of lower power for longer time periods resulted in rigid foams with more beneficial properties (Figure 43)
Figure 43. Thermal conductivity and apparent density of polyurethane foams.
Reproduced with permission from Prociak, A.; Bogdał, D.; Pielichowski, J.; Dziadczyk, J. In Rigid Polyurethane Foams Blown under Microwave Irradiation, Proceedings of the 8th International Conference “Blowing Agents and Foaming Processes 2006, Munich”, 2006.108
In general, better mechanical properties were observed with the increase of apparent density of rigid polyurethane foams. However, in this case, it must be stressed that an increase of apparent density of about 20% increases compressive strength by more than 100% (Figure 44).
Figure 44. Apparent density and compressive strength of polyurethane foams measured in parallel and perpendicular rise directions.
Reproduced from Bogdal, D.; Prociak, A.; Pielichowski, J. In Application of Microwave Irradiation for Chemical Recycling of Polyurethanes, Proceedings of Global Symposium on Recycling, Waste Treatment and Clean Technology, REWAS, Madrid, 2004.109
Eventually, the application of microwave irradiation for blowing of polyurethane foams led to reduction in the amount of amine catalysts required by half. The possibilities of the application of partially decomposed cellular polyurethane waste under microwave irradiation to formulate modern ecological systems for manufacturing heat-insulating foams were also presented.109
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780444533494001217
Vitamin D Binding Protein
Marijn M. Speeckaert, … Joris R. Delanghe, in Advances in Clinical Chemistry, 2014
6.7 Pulmonary diseases
6.7.1 Asthma
Little is known about the role of DBP in children with asthma. A small cross-sectional study showed significantly higher DBP levels in bronchoalveolar lavage fluid of children with severe therapy-resistant asthma as compared with those in moderate asthma and control individuals. DBP levels in bronchoalveolar lavage fluid correlated with asthma severity, as assessed by the asthma control test, spirometry, and usage of inhaled corticosteroids. Excessive DBP in the airway may limit vitamin D bioactivity with potential immunological consequences. Due to the stimulated chemotactic functions of monocytes and neutrophils and together with the macrophage-activating factor, DBP can drive excessive airway alveolar macrophages into a more inflammatory state, diverting them from their tolerogenic role. However, a raised airway DBP level can also be a reflection of a chronic inflammation in the airway [158].
Isocyanate-induced occupational asthma is the most frequent cause of occupational asthma worldwide. Proteomic analysis with bronchoalveolar lavage fluid showed an upregulation of DBP expression in patients with diphenyl-methane diisocyanate occupational asthma [159]. Serum DBP level may be used as a useful serological marker for the detection of isocyanate-occupational asthma among workers exposed to isocyanate and for prediction of the severity of airway hyperresponsiveness, with a sensitivity of 69% and specificity of 81% [160]. DBP is associated with immune modulation of T helper 2-mediated inflammation, influencing the susceptibility to asthma [161]. In addition, the presence of megalin in the alveolar epithelial cells may mediate the endocytic uptake of 25-hydroxyvitamin D-DBP complexes. After uptake, 25-hydroxyvitamin D dissociates from DBP and is metabolized to 1,25-dihydroxyvitamin D [162]. Exposition to toluene diisocyanate may lead to higher DBP levels in bronchoalveolar fluid by suppressing megalin expression in lung alveolar cells [160].
In a Chinese case–control cohort of asthma consisting of 467 cases and 288 unrelated healthy controls, the DBP SNPs rs4588 and rs7041 were significantly associated with the risk of asthma and the DBP1 allele might confer a protective effect (OR: 0.65). No significant association with serum 25-hydroxyvitamin D levels was found, which could be explained by the influence of several genetic loci on the 25-hydroxyvitamin D concentration [163].
6.7.2 Chronic obstructive pulmonary disease
Chronic obstructive pulmonary disease (COPD) is associated with low serum levels of 25-hydroxyvitamin D, which are correlated with forced expiratory volume in 1 s (FEV1) and forced vital capacity (FVC) [164,165]. Genetic association studies have demonstrated a link between the DBP gene and COPD-related phenotypes [166]. DBP has the potential to influence the respiratory function by determining vitamin D bioavailability and via direct effects on innate cell function (neutrophil chemotaxis and macrophage activation). More specifically in the presence of high serum DBP levels, a decreased lung function, expressed as a lower FEV1, has been observed (which is in contrast to the vitamin D status) and high airway DBP concentrations are associated with an increased macrophage activation [167].
Differences in vitamin D level are determined by the underlying DBP genotypes and subjects with a DBP2 allele exhibit a decreased risk of COPD. Although the DBP1F allele has no effect on the age of onset of COPD, the annual decline in FEV1 is significantly higher in Asian subjects [168,169]. The DBP1S variant is not associated with COPD in any racial population [170]. Although variants in the sugar structure of DBP have been proposed to explain the observed differences between the DBP phenotypes, differences in conversion to the macrophage-activating factor are probably much more important [171]. DBP2 is less able to be converted to DBP-MAF, resulting in a reduced macrophage driven parenchymal inflammation and destruction [167]. The protective effect of DBP2 has been confirmed in the Han population of north-east China [172]. However, α1-antitrypsin deficiency patients carrying a DBP2 allele have an increased risk of bronchiectasis, which could be explained by a reduced ability to defend the airway against pathogens [167]. Significant associations have been seen between SNPs rs17467825 and rs1155563 of the DBP gene and FEV1% predicted and FEV1/FVC [166].
6.7.3 Cystic fibrosis
Cystic fibrosis is an autosomal recessive condition, characterized by a vicious circle of progressive inflammation of lung and pancreas, leading to malnutrition and early death. DBP has been proposed as a sensitive marker (T½ = 2.5 days) for nutritional status (especially for lipids), being an integral part of an overall assessment program in cystic fibrosis [173]. Significantly lower mean DBP concentrations have been reported in the sera of cystic fibrosis patients, compared with heterozygotes and controls [173,174]. Lower serum DBP values may point toward a lower lipid absorption or hypolipoproteinemia. In cohort study of 116 cystic fibrosis patients, a strong correlation between serum DBP concentration and lipid parameters [serum total cholesterol, low-density lipoprotein (LDL)-cholesterol, triglycerides, and leptin concentration] was demonstrated. This finding was supported by precipitation experiments of VLDL and LDL, which showed a partial coprecipitation of DBP, VLDL, and LDL particles. This suggests a partial presence of DBP as an LDL- and VLDL-bound plasma protein. In a similar way, serum DBP concentration correlated with serum α-tocopherol concentration. Decreased vitamin E levels and a disturbed fat malabsorption are observed in cystic fibrosis. Serum α-tocopherol strongly correlated with total lipids and serum β-lipoprotein concentrations, as it is mainly transported through VLDL and LDL particles [175].
6.7.4 Tuberculosis
In a case–control study (544 adult tuberculosis patients, 400 controls), the DBP2-2 phenotype was strongly associated with susceptibility to active tuberculosis in Gujarati Asians, compared with the DBP1-1 group (OR 2.81, 95% CI: 1.19–6.66; p = 0.009). However, this association was only observed in patients with profound vitamin D deficiency and was lost when the serum 25-hydroxyvitamin D concentration was > 20 nmol/L [176]. Vitamin D deficiency decreases antimycobacterial immunity [177] and vitamin D-deficient carriers of the DBP2 allele may have particularly low circulating concentrations of the 25-hydroxyvitamin D–DBP complex [2]. Carriage of the DBP2 allele was also associated with increased purified protein derivate of tuberculin-stimulated IFN-γ release in Gujarati Asian tuberculosis contacts. This finding suggests that in comparison with DBP2 allele carriers, DBP1 homozygotes are relatively resistant to acquisition of latent tuberculosis infection. The association might be consistent with the reduced ability of DBP2 to convert DBP to DBP-MAF. No association between the DBP phenotype and susceptibility to tuberculosis was observed in the other studied populations in Rio de Janeiro, Cape Town [176], Kuwait [211], India [212], which had a lower DBP2 frequency and a low prevalence of vitamin D deficiency [176].
6.7.5 Acute lung injury
Diffuse alveolar damage with neutrophils, macrophages, and protein-rich edema fluid in the alveolar spaces, together with capillary injury and disruption of the alveolar epithelium are the pathological characteristics of acute lung injury [171,178]. Although there is a lack of candidate gene studies relating to the vitamin D axis, patients with adult respiratory distress syndrome exhibited decreased serum DBP concentrations [179]. The massive cellular injury is associated with the liberation of large amounts of actin in the extravascular space and with an increased formation of DBP–actin complexes [171].
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780128000946000017
Polymer Characterization
P.L. Rinaldi, … E.B. Twum, in Polymer Science: A Comprehensive Reference, 2012
2.06.4.2.5 Applications of 2D- and 3D-NMR to the study of polymer chain ends and block junctions
2.06.4.2.5(i) Polyester urethane
LeMaster et al.68 made very elegant use of 1D-, 2D-, and 3D-NMR data to study the structure of a linear polyester urethane (Estane 5703), synthesized via the reaction of 1,4-butanediol with 4,4′-diphenylmethane diisocyanate and adipic acid, 42. There are four types of butanediol groups in the backbone, those with: two ester linkages (proximal and distal, e-e), two urethane linkages (proximal and distal, u-u), proximal ester and distal urethane linkages (e-u), and proximal urethane and distal ester linkages (u-e). In addition, there are two types of terminal –CH2OH groups based on whether the distal –CH2O– group in the butanediol unit is bound in an ester or urethane linkage.
From an 1H{13C} HSQC 2D-NMR experiment they were able to extract the regions of the spectrum shown in Figure 39. Figure 39(a) contains the region of the spectrum produced by backbone –CH2OR groups. They reasoned that ester CH2O– groups should be upfield and assigned these structures to the two high-field crosspeaks (upper right); that is to the methylenes of ester linkages (e groups). They assigned the two downfield crosspeaks (lower left) to the methylenes of urethane CH2O– linkages (u groups). Within each of these regions two crosspeaks are observed, one for a distal e linkage and one for a distal u linkage. They relied on 3D-NMR to provide unequivocal proof of these resonance assignments (see below).
Figure 39. Two regions from 1H–13C 2D-HSQC spectrum of commercial polyester urethane, Estane 5703: (a) region showing –CH2–OR methylene resonances from internal butanediol units, and (b) region showing –CH2–OH methylene resonances from terminal butanediol units.
Reprinted with permission from LeMaster, D. M.; Hernandez, G. Macromolecules 2000, 33, 3569.68 Copyright 2000 American Chemical Society.
Figure 39(b) contains the region from the HSQC 2D-NMR spectrum showing correlations from the –CH2OH chain ends of 42. The much weaker crosspeak intensities (relative to those in Figure 39(a)) were consistent with these signals arising from chain-end structures. They postulated that the chemical shift order within this group should be the same as that found for the internal –CH2O– groups of 1,4-butanediol units, but again they relied on 3D-NMR data to prove their assignments. To verify different structures from internal as well as terminal butanediol units present in the polymer, they used the extensive spin-coupling correlations from 3D 13C-purged 1H–1H–13C TOCSY-HSQC; these data are presented in Figures 40 and 41.
Figure 40. Expansions from the 3D 13C purged 1H–1H–13C TOCSY-HSQC spectrum of Estane 5703 showing 1H-1H slices, at two different 13C shifts, (a) 63.2 and (b) 60.2 ppm, containing diagonal resonances from the internal butanediol units.
Reprinted with permission from LeMaster, D. M.; Hernandez, G. Macromolecules 2000, 33, 3569.68 Copyright 2000 American Chemical Society.
Figure 41. Overlay of two expansions from the 3D 13C purged 1H–1H–13C TOCSY-HSQC spectrum of Estane 5703: 2D 1H–1H slices at 13C shift of 63.2 (blue) and 60.2 (green) ppm.
Reprinted with permission from LeMaster, D. M.; Hernandez, G. Macromolecules 2000, 33, 3569.68 Copyright 2000 American Chemical Society.
Shown in Figure 40 are two 2D 1H–1H slices from 3D TOCSY-HSQC spectrum at different 13C chemical shift of 63.2 (Figure 40(a)) and 60.2 ppm (Figure 40(b)). The former corresponds to a segment from the TOCSY plane at the chemical shift of 13C attributed to e-e CH2O groups, and the latter corresponds to a segment from the TOCSY plane at the chemical shift of 13C from e CH2OH groups. Ordinarily, the 3D-TOCSY-HSQC spectrum will contain TOCSY planes at the shifts of each proton-bearing carbon. These TOCSY planes will contain a diagonal peak in which the proton chemical shifts correspond to the shift of the 1H attached to that 13C, and off-diagonal crosspeaks from other 1H atoms that are part of the same 1H spin system. In the case of symmetric structures such as butanediol, it becomes impossible to distinguish a diagonal peak as the original source of magnetization for TOCSY transfer, from a crosspeak arising because the spin is the destination of TOCSY transfer down the chain of coupled spins.
In the version of the 3D TOCSY-HSQC experiment used by LeMaster and co-workers, they suppress the signals from the source 1H–13C pair, which usually produces diagonal peaks in the 1H–1H TOCSY dimension. Since they did not decouple 13C in the f1 dimension, the trace doublet flanking the intense crosspeak (at 4.0/4.0 ppm) at the bottom of Figure 40(a) is the residual signal from incomplete suppression of magnetization derived from e-e 13CH2O groups. The doublet confirms the identity of this signal component in the 3D-NMR spectrum. The strong signal at 4.0/4.0 ppm is the consequence of signal initially derived from e-e CH2O– groups, and ultimately transferred through TOCSY mixing to e-e 13CH2O– groups at the other end of the internal butanediol unit (symmetric in all respects except for isotopic distribution). The strong crosspeak at (4.0, 1.6) ppm in Figure 40(a) is a signal produced by the magnetization transfer from two equivalent central methylene units of the internal butanediol to methylene group of –CH2–OR of the butanediol unit.
The much weaker crosspeaks in Figure 40(b) were attributed to the lower occurrence of unsymmetrical CO2CH2CH2CH2CH2OH chain-end groups. This region of the TOCSY plane was taken from the 3D spectrum, at the 13C chemical shift (60.2 ppm) of the terminal e –13CH2OH group. The residual doublet from incomplete suppression of 13CH2OH source magnetization (3.4/3.4 ppm) is not detected in this plot due to its much weaker intensity (lower occurrence of chain-end vs. backbone butanediol units). At the 1H chemical shift of 3.4 ppm, crosspeaks are observed to 4.0, 1.6, and 1.45 ppm. These were attributed to TOCSY transfer to CH2 groups α, β, and γ to the ester group, respectively.
Evidence for magnetization transfer within symmetric and asymmetric butanediol units are shown in Figure 41, where two 1H–1H TOCSY slices at different 13C chemical shifts were overlaid. The peaks in blue are from the f2 plane at δC = 63.61 ppm and are attributed to urethane linked CH2O groups; while those in green are from the f2 plane at δC = 63.24 ppm and are attributed to ester linked CH2O groups. The crosspeaks at (4.01, 4.01) and (4.09, 4.09) are derived from symmetric diester and diurethane-linked butanediol units, respectively, as the 1H chemical shifts are the same in both the f1 and f3 dimensions. The crosspeaks at (4.04, 4.07) and (4.07, 4.04) are derived from asymmetric ester-urethane linked butanediol units.
The ultimate objective of the project was to measure the composition of 42 and to study the reaction kinetics of its hydrolysis. Since all of the resonances of this complex polymer were not resolved in the 1D-NMR spectra, these researchers resorted to integration of the crosspeak volumes in the 2D-HSQC spectra. They noted that a number of factors normally impede the use of 2D-NMR data for quantitative analysis. These included (1) variable polarization transfer efficiency due to a range of J couplings used for INEPT polarization transfer in the various stages of the HSQC pulse sequence; (2) resonance offset effects; (3) a range of 1H T1 causing differential saturation during the relaxation delay between transients; and (4) differential polarization transfer efficiencies due to variations in 1H and 13C T2’s. To this list, the effects of limited digital resolution on the accuracy of peak volume measurement must also be considered. The effects of digital resolution can easily be dealt with by increasing the number of sampled data points in the f1 and f2 dimensions, and extending the data set using numerical methods such as linear prediction and by zero filling.
The authors dealt with J coupling issue by comparing crosspeak intensities for CH2 groups, which have a narrow range of couplings. They also noted that for this polymer, the range of chemical shifts was small 1.5 kHz for 13C, compared to an 18.5 kHz for the B1 field created by the pulse. Thus, resonance offset errors were insignificant for this system. To correct for shorter 1H T1’s of the backbone relative to the chain-end groups, they collected two HSQC spectra with different relaxation delays. Based on the changes of the intensity ratios for the CH2OR/CH2OH they derived a 1.2 s difference in T1’s and calculated a corrected intensity ratio, from volume integrals of peaks similar to those seen in Figure 39. They were less successful in deriving T2 values and their influence on 2D-NMR peak volumes, but did estimate a small signal loss from T2 relaxation of the backbone and chain-end groups. They estimated less than 10% error in their calculations.
Using the 2D-HSQC peak volumes from backbone e-e, e-u, u-e, and u-u –CH2–OR correlations in Figure 39(a) and from chain-end e– and u–CH2–OH correlations in Figure 39(b), they derived an internal –CH2–OR/terminal –CH–OH peak volume ratio of 203:1. Similarly, the direct integration of crosspeak volumes in the spectrum shown in Figure 39(a) gave the monomer distribution ratio of 4.76:3.76:1.00 for butanediol:adipate:diphenylmethane units.
Arylamine chain-end groups were quantified by direct integration of the well-resolved 1H 1D-NMR resonances from arylamine end groups. The resonances from –CH2–COOH methylene chain-end groups were found to overlap with the tail of large –CH2–COOR resonances in both the 1D- and 2D-NMR spectra (spectra not shown); the content of free acid end groups in the unhydrolyzed sample could not be determined independently from the NMR data. Ideally, the intensity increase between time points in the hydrolysis series should be identical for both the butanediol –CH2OH group and –CH2COOH group during solid-state hydrolysis of the polymer; the free –CH2COOH end group in the unhydrolyzed sample was estimated as 10% of total end groups. From this, they were able to determine the ratio of hydroxyl/acid/arylamine end groups as 86.3/10/3.7. Having all these data, in hand, the M¯n of the starting polymer was estimated to be 40.2 kDa.
By measuring volume integrals from 2D-HSQC solution spectra of reaction aliquots, collected over a reaction time of 49 days, they were able to do an impressive job of monitoring the reaction kinetics for solid-phase hydrolysis of 42 at elevated temperature.
Read full chapter
URL:
https://www.sciencedirect.com/science/article/pii/B9780444533494000248
Interstitial Lung Disease: Nonidiopathic Interstitial Pneumonia
Moisés Selman MD, in Clinics in Chest Medicine, 2004
Although HP is classically associated with antigen (protein/micro-organism) exposure, inhalation of some low molecular weight chemical compounds also may provoke the disease. Workers exposed to toluene diisocyanate and diphenylmethane diisocyanate in the plastic manufacturing process, painting, and electronics industry may develop HP [28,29]. Diisocynates contain the highly reactive isocyanate; various products, especially urethane resin, are made from these chemicals. A wide spectrum of respiratory disorders has been described as a result of exposure to diisocyanates, including bronchitis, asthma, and HP. The mechanisms involved in chemical-induced HP are still unclear, but it seems that diisocyanates may conjugate with some human proteins, such as serum albumins, and then elicit a humoral and cellular immune response. IgG and IgA antibodies to diisocyanates have been detected in patients with diisocyanate-induced HP, and circulating lymphocytes proliferate and express various proinflammatory cytokines in the presence of diisocyanates [30].
Read full article
URL:
https://www.sciencedirect.com/science/article/pii/S0272523104000450
Acute and subacute chemical-induced lung injuries: HRCT findings
Masanori Akira, Narufumi Suganuma, in European Journal of Radiology, 2014
12 Granulomatous pneumonitis and hypersensitivity pneumonitis
Cobalt, titanium, aluminum, copper, talc, and glass fibers have been reported to cause granulomatous pneumonitis with sarcoid-like granuloma [40–42]. Organic isocyanates, such as toluene diisocyanate and diphenylmethane diisocyanate, have been associated with hypersensitivity pneumonitis [43]. The characteristic HRCT features of hypersensitivity pneumonitis are centrilobular nodules, ground-glass opacities, and mosaic perfusion pattern [44]. Subacute hypersensitivity pneumonitis is one of the most common diseases that manifest centrilobular ground-glass nodules without associated tree-in-bud opacities. In the prominent symptomatic period, airspace consolidation and ground-glass opacities are prominent and micronodules are fine and ambiguous on CT (Fig. 11).
Fig. 11. Images in a 64-year-old man who had inhaled an insecticide. The subject suffered from acute respiratory distress syndrome after inhalation of an insecticide. (a) Chest radiograph obtained on the day of exposure shows consolidation distributed diffusely throughout both lungs, with peripheral predominance. Small nodular-like opacities are seen in both upper lungs. Interlobar pleural effusion is evident in the right lung. (b) Transverse HRCT scan shows mixture of ground-glass opacity and consolidation, and interlobular septal thickening. A few small nodular opacities are evident (arrows). (c) Histologic specimen obtained by surgical lung biopsy shows noncaseating granuloma surrounding the terminal bronchiole and its adjacent arteriole.
Read full article
URL:
https://www.sciencedirect.com/science/article/pii/S0720048X14002241
|
|
В этой статье не хватает ссылок на источники информации.
Информация должна быть проверяема, иначе она может быть поставлена под сомнение и удалена. |
| 4,4′-метилендифенилдиизоцианат | |
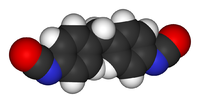 |
|
| Общие | |
|---|---|
| Систематическое наименование | 1-изоциано-4-[(4-изоцианофенил)метил] бензол |
| Сокращения | МДИ, MDI |
| Традиционные названия | Чистый МДИ, Мономерный МДИ |
| Химическая формула | C15H10N2O2 |
| Физические свойства | |
| Состояние (ст. усл.) | бесцветные или слегка желтые кристаллы |
| Молярная масса | 250.25 г/моль |
| Плотность | 1.230 г/см³ |
| Термические свойства | |
| Температура плавления | 40 °C |
| Температура кипения | 190 °C |
| Классификация | |
| Рег. номер CAS | 101-68-8 |
| Рег. номер PubChem | 7570 |
| SMILES | O=C=Nc1ccc(cc1)Cc2ccc(N=C=O)cc2 |
| Безопасность | |
| Токсичность | относительно низкая |
Метилендифенилдиизоцианат (дифенилметандиизоцианат) — общеизвестный как МДИ, является ароматическим диизоцианатом. Существует в виде трех изомеров: 2,2′-МДИ, 2,4′-МДИ и 4,4′-МДИ, но 4,4′-изомер является наиболее широко используемым. Этот изомер также известен как чистый МДИ (мономерный МДИ, ММДИ, pure MDI, MMDI). Синонимы: 4,4′-диизоцианатодифенилметан, метилен-бис-(4-фенилизоцианат).
МДИ совместно с полиолом является сырьем для производства полиуретанов. На производство МДИ приходится около 60 % мирового рынка изоцианатов по состоянию на февраль 2011 г.[1]
Содержание
- 1 Виды МДИ
- 2 Физические свойства
- 3 Производство
- 4 Реакционная способность
- 5 Применение
- 6 Безопасность
- 7 См. также
- 8 Примечания
- 9 Ссылки
Виды МДИ
Коммерчески используются несколько видов МДИ, в том числе чистый МДИ (основа — 98 % 4,4′-МДИ),[1] сырой МДИ (полимерный МДИ, ПМДИ, crude MDI, PMDI). Название «полимерный МДИ» не вполне соответствует действительности. ПМДИ не является полимером. Сырой МДИ представляет собой смесь продуктов, содержащую обычно 55 % МДИ (2,4′-и 4,4′-изомеры), 25 % триизоцианатов и 20 % полиизоцианатов.[2] В России сырой МДИ называют Полиизоцианат, ПИЦ, «компонент В».
Физические свойства
Мономерный МДИ представляет собой бледно-желтое твердое вещество с температурой плавления 37-38 °C. Сырой МДИ является обычно жидкостью от желтого до коричневого цвета с характерным запахом плесени.
Производство
По состоянию на февраль 2011 года мировые мощности по производству МДИ составляют 5,8 млн тонн в год.[1] Крупнейшие производители BASF, Bayer, Huntsman, Dow, Mitsui Chemicals, Nippon Polyurethanes и Yantai Wanhua, а также BorsodChem (которая с начала 2011г является частью Yantai Wanhua[1] (англ.)). Все основные производители МДИ являются членами Международного Института Изоцианатов (International Isocyanate Institute), целью которого является содействие безопасному обращению с МДИ и ТДИ на рабочих местах, в обществе и окружающей среде.
МДИ получают фосгенированием соответствующих дифенилметандиаминов. Изомерное соотношение МДИ определяется изомерным составом дифенилметандиаминов. Перегонкой полученной смеси получают сырой МДИ (смесь олигомерных полиизоцианатов), а также смесь изомеров МДИ с низким содержанием 2,4′-изомера. Дальнейшая очистка представляет собой фракционирование смеси изомеров МДИ.[3]
Реакционная способность
Положение изоцианатных групп влияет на их реакционную способность. В 4,4′-МДИ, две изоцианатные группы эквивалентны, однако в 2,4′-МДИ они обладают различной реакционной способностью. Группа в положении 4 примерно в четыре раза более реакционноспособна, чем группа в положении 2.[4]
МДИ, являясь электрофилом, реагирует с нуклеофильными веществами, такими как вода, спирты, кислоты, амины и т. п.
Применение
Основное применение МДИ — производство жестких полиуретановых пен. МДИ является «компонентом Б» системы, в которой реагируя с «компонентом А» образует полиуретан. Для вспенивания получившегося полимера применяют различные низкокипящие вещества, например фреон или пентан. Полученный пенополиуретан (ППУ) обладает высокими теплоизолирующими свойствами, которые и определяют его основное применение: строительная теплоизоляция (сэндвич-панели, декоративные панели, напыляемая изоляция, монтажная пена), холодильное оборудование, изоляция труб и др.
Также МДИ применяется для строительных адгезионных материалов (герметиков, клеев и т. п.)
Безопасность
МДИ является наименее опасным из общедоступных изоцианатов, но не является безопасным.[5] Очень низкое давление пара снижает его опасность во время обращения по сравнению с другими основными изоцианатами (ТДИ, ГДИ). Тем не менее, он, как и другие изоцианаты, является аллергеном и сенсибилизатором. У некоторых людей может развиваться чувствительность к изоцианатам даже при воздействии чрезвычайно малых концентраций, имеющая опасные последствия, в том числе астматические реакции. Обращение с МДИ требует строгого инженерного контроля и средств индивидуальной защиты. По сравнению с другими органическими изоцианатами, МДИ имеет относительно низкую токсичность для человека.
См. также
- Полиуретаны
- Изоцианаты
- Монтажная пена
Примечания
- ↑ 1 2 3 Евразийский химический рынок № 2 (77), 2011
- ↑ Материалы сотрудничества РХТУ им. Д. И. Менделеева и Dow Chemicals
- ↑ Christian Six and Frank Richter «Isocyanates, Organic» in Ullmann’s Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. DOI:10.1002/14356007.a14_611
- ↑ Randall David The Polyurethanes Book. — New York: Wiley, 2002. — ISBN 0-470-85041-8
- ↑ Allport DC, Gilbert, DS and Outterside SM (eds) (2003). MDI and TDI: safety, health & the environment: a source book and practical guide. Chichester, Wiley.
Ссылки
- Первый Российский портал, посвященный изоцианатам
- International Chemical Safety Card 0298
- ISOPA — European Diisocyanate and Polyol Producers Association
- International Isocyanate Institute
- MDI and the Environment — 2005 presentation by Center for the Polyurethanes Industry
- NIOSH Safety and Health Topic: Isocyanates, from the website of the National Institute for Occupational Safety and Health (NIOSH)
- Concise International Chemical Assessment Document 27
- Карточка безопасности МДИ на странице «Института промышленной безопасности, охраны труда и социального партнерства»
- Инфомация по безопасности на Xumuk.ru
| Содержание изоцианатных групп, %: | 31.33 |
| Вязкость, Па•с/25оС: | 172 |
| Кислотность, %: | 0.0074 |
| Относительная плотность ( при 25оС): | 1.234 |
Полиизоцианат (Метилендифенилдиизоцианат (МДИ), дифенилметандиизоцианат) — ароматический диизоцианат.
Общеизвестен в виде трех изомеров: 4,4-МДИ — наиболее широко используемый изомер, также известен как чистый МДИ и 2,2-МДИ, 2,4-МДИ.
Полиизоционат это темно-коричневая жидкая смесь 4, 4 — дифенилметандиизоцианата (MDI) с изомерами и гомологами.
В производстве пенополиуретана (ППУ) МДИ чаще обозначается как «компонент Б» системы, образующей полиуретан реагируя с «компонентом А».
Хим.формула C15H10N2O2
Синонимы и международное название
полиметан полифенил изоцианат, полиметилен полифенил изоцианат, дифенилметандиизоцианат, полимерный дифенилметан диизоцианат, дифенилметан-4.4′-диизоцианат, 4,4′-диизоцианатодифенилметан, 1-изоциано-4-[(4-изоцианофенил)метил] бензол, компонент В.
Применение
1) в производстве пенополиуретанов, пенолиуретановых пен, клеев, лаков и покрытий
2) в производстве покрытий CASE (Coating, Adhesives, Sealants, Elastomers) адгезивов, герметиков и эластомеров
3) в производстве полиуретановых полов
4) в производстве преполимеров, уплотнительных материалов
Упаковка
Металлические бочки по 250 кг.
С этим товаром используется:
— ДИОКСИД ТИТАНА
— Стеариновая кислота MASFLEX
— Трихлорэтилфосфат (ТХЭФ)
— Трихлорпропилфосфат
Реагент делится на различные типы, в зависимости от сферы применения:
- Толуилендиизоцианат ТДИ
- Метилендифенилдиизоцианат МДИ.
В свою очередь ТДИ делится на такие изомеры как 2,4-ТДИ и 2,6-ТДИ. И применяется в основном для получения эластомеров, как реагент в лабораториях, а также для производства искусственной кожи.
Чтобы купить оптом с доставкой до Перми или Калининграда нужно:
— свзяаться сменеджером,
— предоставить карточку партнера,
— получить коммерческое с ценой,
— составить договор и согласовать сроки поставки.
Поставляется реагент точно в срок. При этом вы можете выбрать варант пробной поставки с сертификатами или сразу заказать нужное количество.
Одно можно сказать точно — Русимпэкс поставляет только качественную промышленную химию, которая проверяется производителем и независимой лабораторией (для 100% уверенности в качестве).
Доставка и оплата
Чтобы 4,4 — дифенилметандиизоцианат купить оптом от производителя в город Сергиев Посад (московская область) нужно оставить заявку на почту. Указать объем и реквизиты. В течении пары часов вам позвонит менеджер, расскажет о нюансах работы. И после подписания договора, со склада в Тульской области, за 3 часа вам поступит реангент на склад. 4,4-мди купить в Сергиев Посад по лучшей цене легко!




















